Abstract
Background: Extranodal NK/T-cell lymphoma (ENKL) is an EBV-associated malignancy of rare occurrence in Western countries. There is limited data on the survival of patients with ENKL in the United States (US).
Objective: To describe survival of US patients with ENKL, to inquire if the long-term survival has improved in the most recent years, and to evaluate factors possibly associated with survival among ENKL patients.
Design/Method: We used the National Cancer Institute' Surveillance Epidemiology and End Results (SEER-18) database to determine the survival of patients with ENKL. Inclusion criteria was the diagnosis of ENKL occurring at any age, as first malignant neoplasm diagnosed between 2000 and 2018. Follow-up data was available to the end of 2020. Cases were divided in two "eras", 2000-2009 and 2010-2018, with the latter being expected to reflect the use of asparaginase-base chemotherapy regimens. Survival was estimated using the method of Kaplan-Meier. The impact of era, age, race, gender, and stage on survival were accessed utilizing multivariate analysis.
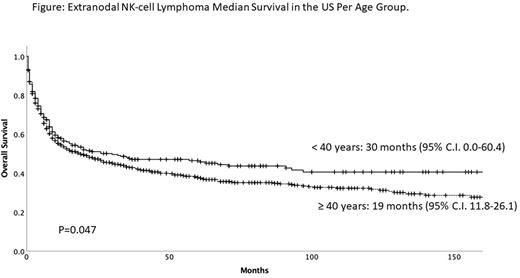
Results: A total of 967 cases were included. Median age of patients was 52 years, 267 (28%) were < 40 at time of diagnosis, 630 (65%) were male and 599 (62%) had early stage disease. There were 430 patients in the 2000-2009 era and 537 patients in the 2010-2018 era. A high proportion of patients was Hispanic (367, 38%) or Asian/Pacific Islander (215, 22%). The median survival for patients < 40 years was 30 months (95% C.I. 0-60.4), and 19 months for patients ≥ 40 (95% C.I. 11.8-26.1, P=0.047) (Figure). Median survival was better for patients diagnosed in the 2010-2018 era (35 months, 95% C.I. 21-48.9 vs. 13 months, 95% C.I. 8.2-17.7, P=0.003). For patients < 40, median survival was 16 months in 2000-2009 and 61 months in 2010-2018 (P=n.s.). For patients ≥ 40 years, median survival improved from 12 to 33 months respectively (P=0.003). Survival improved for those with limited disease (median n.r. vs. 31 months, P< 0.0001), but not for those with advanced stage (6 months in both eras, P=0.3). In multivariable analysis, diagnosis of ENKL in the 2010-2018 era (HR=0.8, 95% C.I. 0.65-0.91, P=0.003), age ≥ 40 (HR=1.2, 95% C.I. 1.0-1.4, P=0.036), and early stage (HR=0.54, 95% C.I. 0.3-0.7, P<0.01) were associated with survival.
Conclusion: Survival improved for patients with ENKL in the US particularly for patients with localized disease. There is urgent need for different treatment strategies for ENKL patients, especially for those with advanced disease.
Disclosures
Costa:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; AbbVie: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Cairo:AbbVie: Consultancy; Nektar: Consultancy; Jazz: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Omeros: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Sanofi: Speakers Bureau; Sobi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal